

We design, qualify, and operate pharmaceutical water - PW, WFI, and pure steam - to globally recognised references (WHO WPU, USP, Ph. Eur., ISPE), so you get reliable quality, cleaner audits, and simpler day-to-day control.
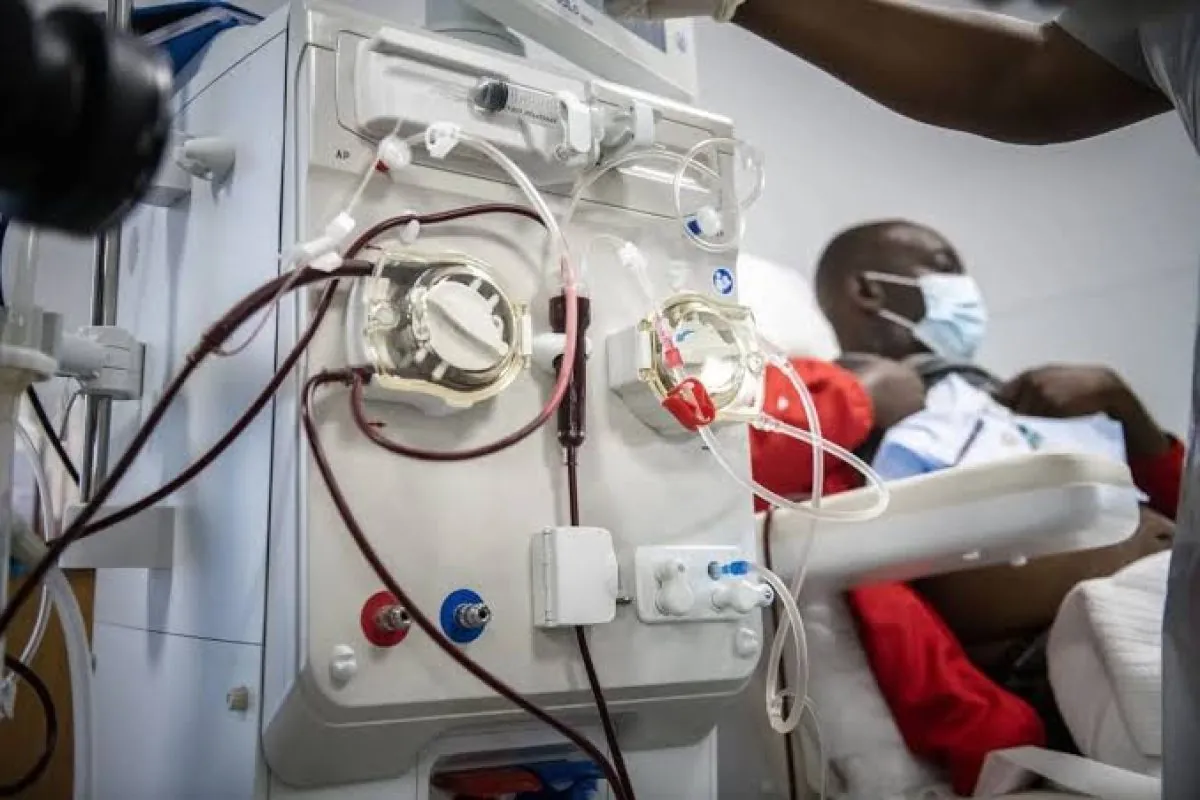
PW, HPW and WFI done right - from URS to validation. We design, build and service BWT/Ecosoft systems with hygienic distribution loops, sanitisation strategies and compliant monitoring, delivered and supported locally across West Africa.
Continuous WFI/HPW with hot and cold loops, 316L orbital-welded distribution, sanitary instrumentation and 21 CFR Part 11-ready data logging - engineered for sterility assurance and rapid, repeatable sanitisation.
![[background image] of rooftop with skyline (for a roofing contractor)](https://cdn.prod.website-files.com/68c5af5fbc9faf057c07e524/68d1a01e4b7c3dc8fa2a9c42_image.jpg)
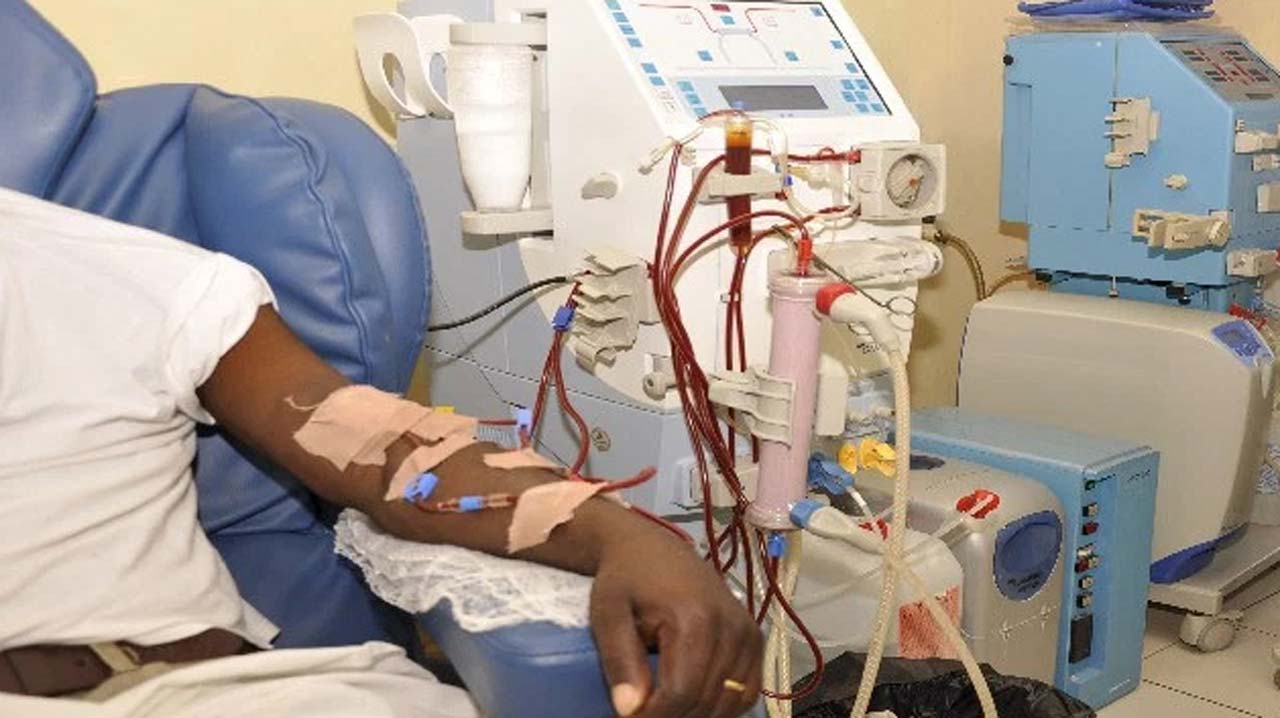
Lab-grade RO/DI and point-of-use polishers for analytical reliability. Stable TOC/conductivity, bacteria control and validated dispensers - backed by calibration, documentation and local service.
Get clear, straightforward info about our services, process, and what to expect when working with us.
We align designs and documentation with cGMP, WHO/ISPE guidance and the major pharmacopeias (USP/EP) for PW/HPW/WFI. Deliverables include URS/DQ, FAT/SAT, IQ/OQ and PQ support.
Yes - where the site and regulator permit, we offer membrane-generated WFI with thermal or chemical sanitisation and full online monitoring. Traditional distillation (MSF/MVC) is also available.
Hygienic design: 316L/electropolished pipework, <3D dead-leg practice, high-velocity loops, sanitary valves, plus thermal/chemical sanitisation and continuous TOC/conductivity/ temperature monitoring.
Complete turnover package: URS, DQ, FAT/SAT reports, IQ/OQ protocols, material and weld logs, calibration certificates, SOP templates and PQ assistance where required.
From compact skids (~0.5–3 m³/h PW/HPW) to larger systems (5-10 m³/h+), with single or dual loops and point-of-use polishers. We size to demand and redundancy targets.
Local technicians, stocked spares, remote monitoring and training. We offer SLAs for preventive maintenance, rapid call-outs and re-qualification support ahead of audits.